All hubs of EXTEDOpulse combine into a complete Regulatory Information Management System (RIMS)
Use the different EXTEDOpulse apps individually or gain additional value by using them together in the cloud
Connected systems work with one another for maximum productivity, efficiency and simplicity
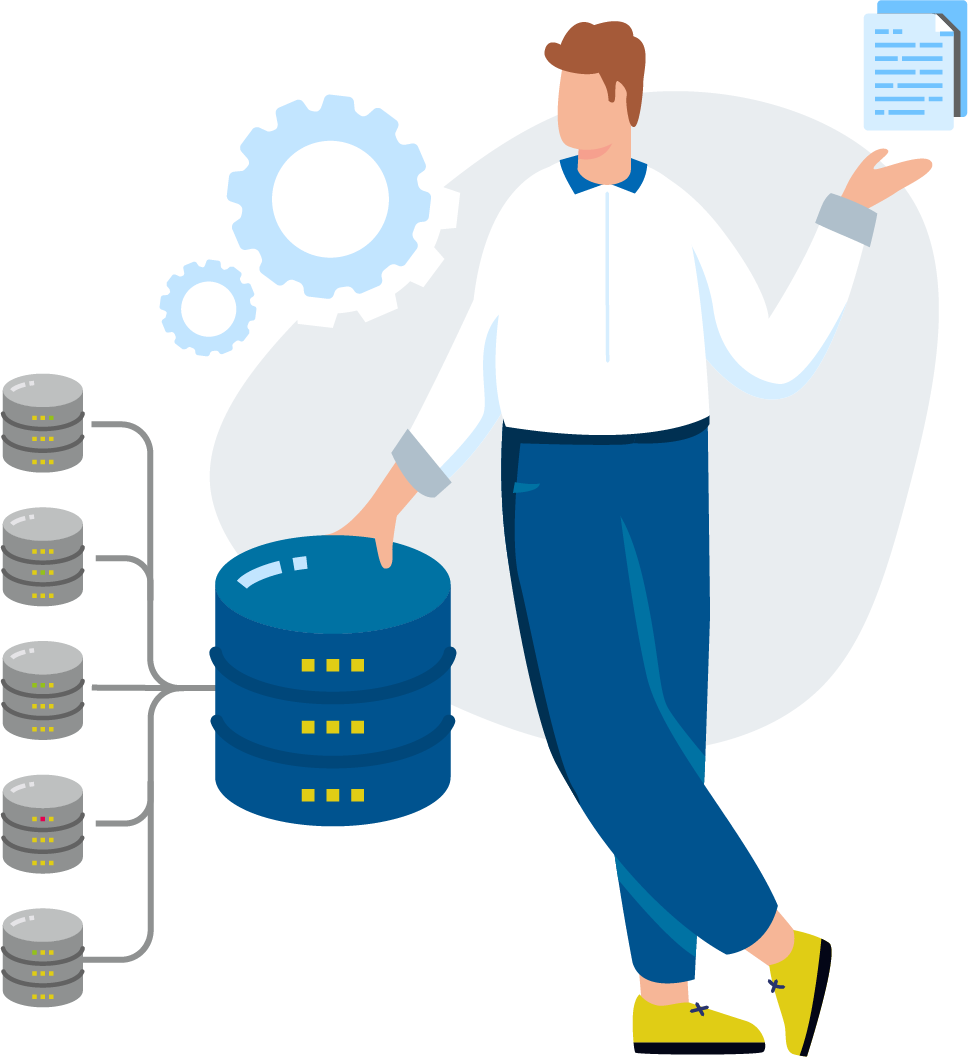
EXTEDOpulse is a comprehensive RIM software solution that addresses every step of pharmaceutical product development. Use the applications individually or gain additional value by using them together based on your requirements.

Developing pharmaceutical products can be a multi-faceted process, involving input and efforts from across an organization. The complexities of operating within a highly regulated industry only further compound the challenges that a life science organization faces with every release. Having our finger on the pulse of the life sciences anatomy allows us to provide you with great synergy, connection and innovation for effortless compliance. Ever-changing regulatory requirements demand maximum flexibility, which we achieve through continuous process improvement and the integration of advanced technologies such as artificial intelligence (AI). EXTEDOpulse has been designed with these aspects in mind to help you connect the dots throughout the entire lifecycle of pharmaceutical products.
EXTEDO understands the complexities of the regulated pharmaceutical product journey. From drug development, to market launch and pharmacovigilance surveillance, EXTEDOpulse is the right solution from the start, for every step. With EXTEDOpulse, the flexibility for better solutions is in your hands. Created from direct feedback and the needs of life science organizations, EXTEDOpulse introduces next-level automation to optimize your team’s productivity across every aspect of development.
Learn more about the EXTEDOpulse Subscription Plans
Master Data Management
The EXTEDOpulse Master Data Management acts as the base layer, seamlessly connected to externally controlled vocabulary repositories such as SPOR, while serving as a virtual resource for all EXTEDOpulse business hubs. The MDM serves as the central repository for maintaining reusable data at a single source of truth.
Document Management Hub
The EXTEDOpulse Document Management Hub supports you in managing regulatory data and documents throughout the entire submission lifecycle, connecting stakeholders with automated processes, eliminating the need to conduct on-site monitoring visits, managing SOP and related training documents, managing CAPA, audits, documenting deviations, and coordinate change control activities, and ensure consistent authoring standards. With EXTEDOpulse, document management becomes an asset for productivity.
Registration Management Hub
EXTEDO’s Registration Management Hub simplifies the process of capturing and managing IDMP, XEVMPD, and other medicinal product information, delivering a single source of truth for product data. Gain a powerful medicinal product database to manage your registrations for effortless compliance. Improve your data quality, increase operational efficiency, and deliver better-automated communication channels between your departments and with the authorities.
Safety Management Hub
The Safety Management Hub enables you to minimize costs and deliver best-practice monitoring and reporting workflows crucial to your business's success. Create, review, submit and maintain multivigilance data and event reports within a single, easy-to-use application. Its future-proof approach is able to generate PSUR, PBRER, and DSUR documentation and is ready for forth-coming standards such as IDMP.
Submission Management Hub
Efficiently build, view, validate, publish, and review compliant submissions based on CTD, eCTD, NeeS, eCopy, IMPD, VNeeS, DMF, ASMF, Clinical Trial Applications, and other submission formats. The Submission Management Hub applications are designed to maintain a comprehensive overview of your regulatory submission statuses across a number of products within multiple different geographic markets. Ensure effortless compliance by using the same validation and reviewing technology as 35 regulatory authorities worldwide, reduce errors, and increase productivity within your organization.
Effortlessly Streamline Your Workflow with Automated Triggering of Applications and Sequences
Using EXTEDOpulse streamlines your submission process with key features like in-progress viewing exports, parallel publishing and reviewing, and efficient submission content planning and tracking. Easily use your submission content plan from the Document Management Hub in the submission structure with drag-and-drop functionality. Synchronize content plans, trigger initial applications and follow-up submissions, and access submission deep links from the Submission Management Hub in the Registration Management Hub.
This approach allows the application to be created once and reused within EXTEDOpulse, reducing time and enhancing data quality and consistency. Triggering the sequence creation directly from the Registration Management Hub with automated links provides easy access from various functional views and ensures a streamlined and efficient workflow.
.png)
Benefit from end-to-end Master Data Management in EXTEDOpulse
Utilizing master data across the regulatory information management process in EXTEDOpulse offers significant advantages. By reusing data, consistency is maintained across all Hubs, significantly reducing the potential for errors. This approach ensures that everyone is working from a single source of truth, streamlining operations and enhancing accuracy. With a unified data set, regulatory compliance becomes more straightforward, and decision-making is based on reliable, consistent information. This not only boosts efficiency but also increases reliability of the regulatory outcomes.
Request your personal EXTEDO Software Demo now!
“EXTEDO is a great example of a company that really understands the requirements of the market.”
Director Business Development, Technical Operations and International Business at Genericon
Some of our Customers





Your plan to effortless compliance
Schedule a call
We’ll discuss your goals and uncover your challenges of operating within a highly regulated industry.
Get a free consultation
Our experienced business and technical team will outline a solution to solve your challenges.
Manage your pharmaceutical product journey effortlessly
Understand the complexities of the regulated pharmaceutical product journey, from drug development, to market launch and pharmacovigilance surveillance.
Contact us
Business Process and Regulatory Consulting Services
Tailored specifically to the needs of regulatory and related stakeholders, EXTEDO’s business process and regulatory consulting services are designed to support you during and after your eCTD submissions. Through a series of workshops, our team of experienced consultants will establish your business needs, understand your processes, and help you to define the most appropriate implementation approach.
Education & Training Services
To ensure you get the most out of your purchased solution, we offer detailed training for each product within the EXTEDOpulse solution portfolio. Training sessions are tailored to your individual needs and cover a broad range of technical and regulatory topics. Designed to educate you on how to utilize your EXTEDO solution, our training sessions are conducted either in-house or onsite.
Technical Consulting
Purchasing a new EXTEDO application is the first step to streamlining business and regulatory processes within your organization. However, ensuring correct installation, implementation and integration is a crucial step in the process of deploying your new solution.
Regulatory Publishing Services
With agencies required to produce ever more submissions in electronic formats, the cost and effort involved in converting paper-based documentation into eCTD, and other electronic formats, and publishing them to the authorities can be prohibitive for many smaller life sciences organizations.








.png)
