

Effortlessly maintain compliance for quality documents and processes
The QMS tool for managing life science quality information & processes in a controlled and automated manner.
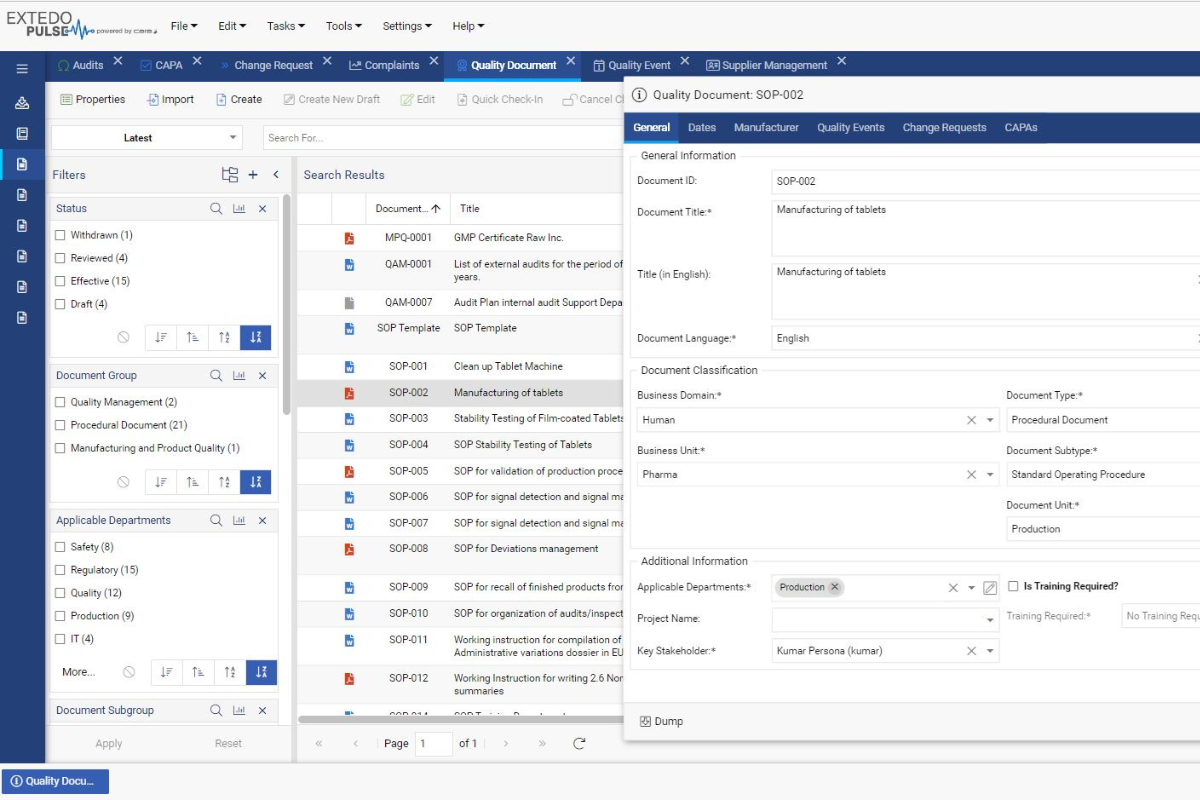
Streamline your management of controlled documents
Track and handle quality processes in a controlled and transparent manner
Enhance efficiency with automated workflows and alerts
A QMS with poor document and process management practices means wasting your money
Managing large numbers of controlled documents and processes within a QMS is complex, requiring significant time and effort.
- I am unable to manage the effort related to audit preparation
- My quality information is stored in disconnected silos with different interfaces
- I am unable to efficiently manage the users in my quality processes
- I don’t know how to share information with 3rd parties safely and correctly
- I do not have full control of my quality information
- KPI management is not available in our current system but is required


Managing controlled documents and quality processes should be effortless.
Empower quality with automation
Use workflows for review, approval, periodic review, and redundancy activities.
Gain quality insights & direction
Provide your team with dashboards for a transparent overview and to consistently improve quality.
Use automation for learning management
Allow the learning management to help you perform trainings in a structured way.
Go beyond quality document management
Create and manage quality events, audits, CAPAs, and change requests as well as your equipment and suppliers.
.png)


"EXTEDO is a great example of a company that really understands the requirements of the market."
Your plan to effortless compliance
Book a meeting
We’ll discuss your challenges and goals with managing your quality processes and controlled documents.
Create a plan
We will outline an approach to simplify the management of your processes and controlled documents.
Take control of your documents
With automated processes, you can effortlessly manage quality documentation and stop worrying about audits.
A solution to manage controlled documents and quality processes for life sciences
Poor document management systems and complicated practices for process management are wasting your time and money.
eDOCSmanager Quality offers a platform for managing quality documentation in a controlled and automated way, allowing you to focus on creating your documents and refining their content rather than managing them.


Request your personal EXTEDO Software Demo now!
Assign automatic quality control workflows
EXTEDO’s QMS tool powered by CARA provides everything you expect from a Quality Management Solution and more. Gain the power to assign automatic quality control workflows while using risk assessment tools to identify and counter threats before they happen. A dashboard functionality keeps you informed about the current status. The solution further includes all standard document creation, review, approval, sign-off, and publishing features, as well as controlled printing for your team to make your quality control processes efficient, accurate and effective.
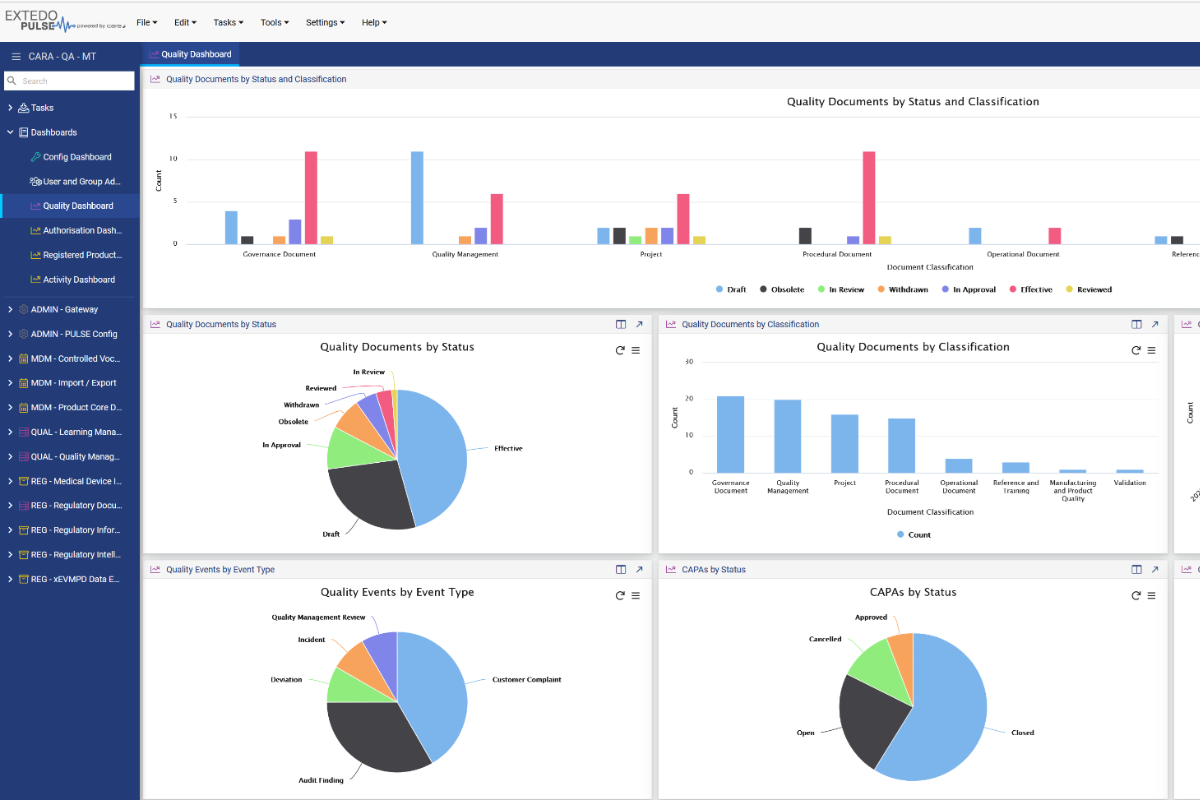
Advanced quality automation
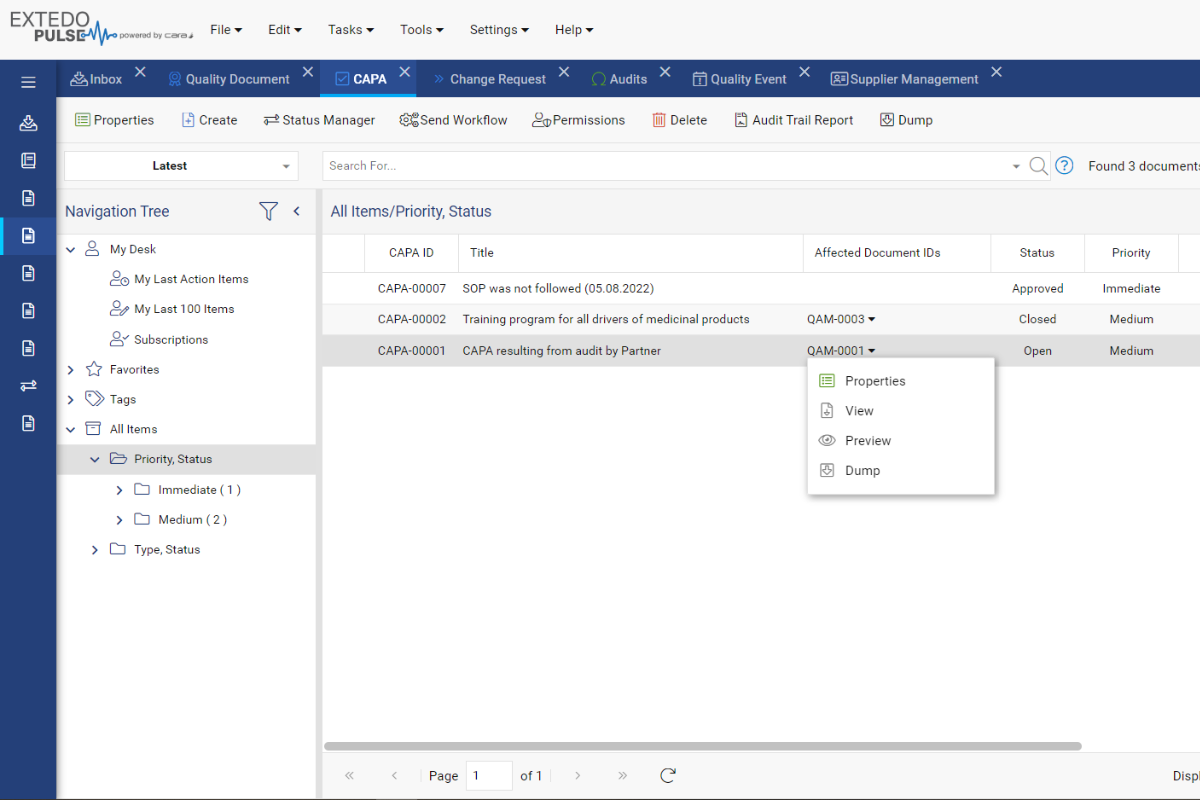
EXTEDO’s solution enables you to incorporate quality into your natural workflow. Easily create internal or external Quality Events for regular quality assurance checks, audits, complaint resolution, and more.
You can even create and manage CAPA items for Quality Events, including sharing with external auditors to allow them to update CAPA info directly.
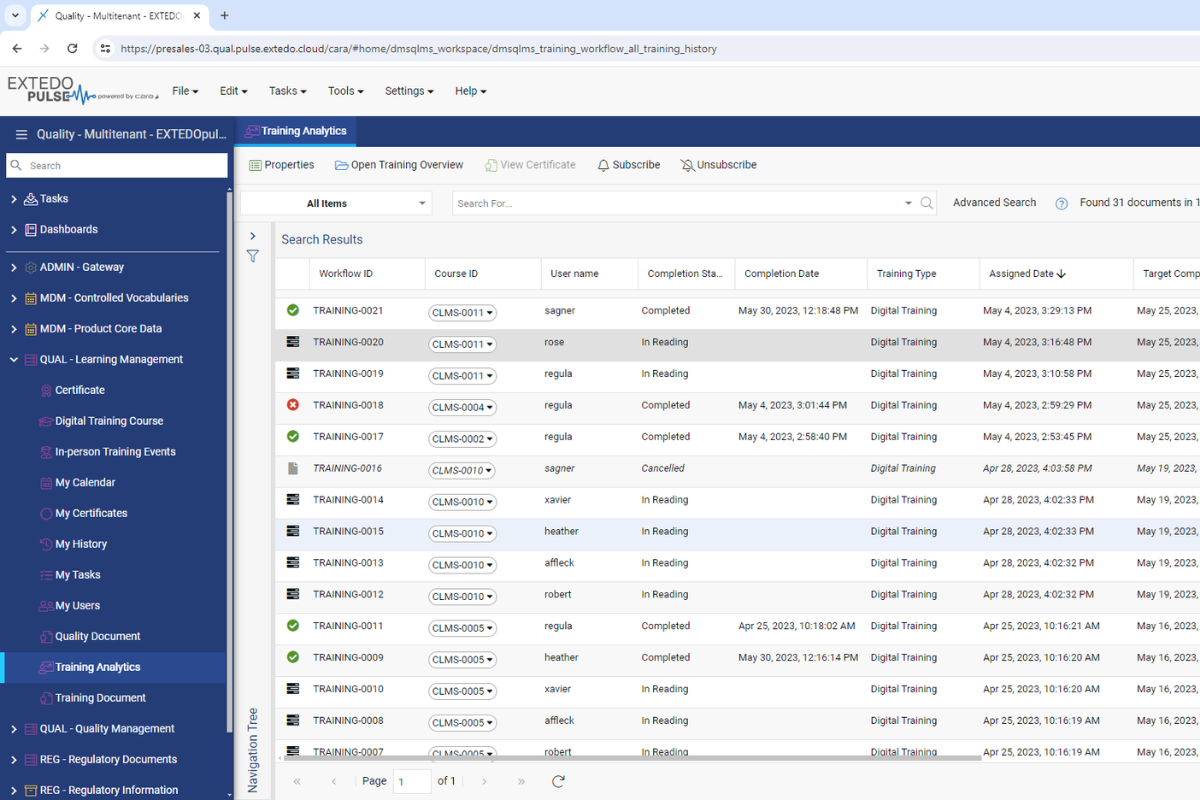
Capture and provide document training from a single, intuitive interface
Automatically manage workflows for the generation, approval, and periodic review of your controlled documents. EXTEDO’s solution enables you to capture and provide document training from a single, intuitive interface. When training efforts are performed, the module records the accomplishment and marks it as completion with certificate attachments and training reports, helping you gain control over the professional development of your team.
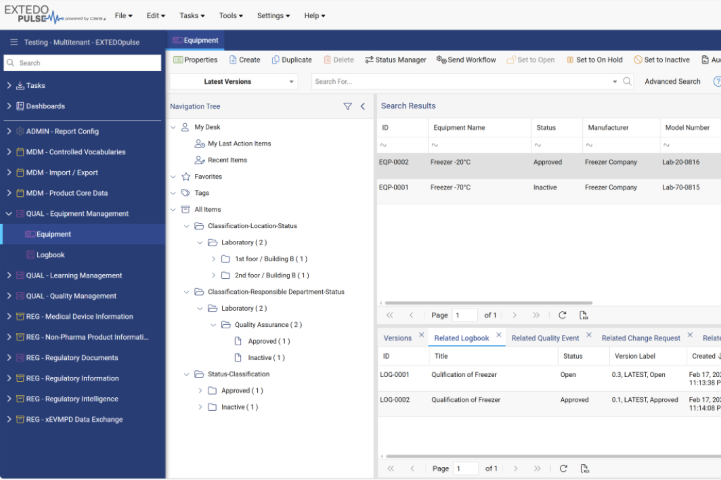
Maintain and monitor your GxP relevant equipment throughout its lifecycle in a transparent way
Integrated equipment management functionalities allow you to track the different lifecycle states of your equipment - from initial qualification to maintenance and final inactivation. The status of every asset is documented in one system, facilitating correct maintenance intervals and effectively reducing costs. Collect, access, and manage detailed information regarding qualification and maintenance activities with the logbook data item, ensuring the efficient and economical operation of your material and thus your entire work procedures.
A solution for every quality challenge
EXTEDO’s solution gives you the tools you need to perform any quality control task from a single location. Well-structured data-capturing masks allow you an easy way to track all information and guide you through the necessary steps of analyzing a Quality event. Simultaneously, helpful Corrective Action and Preventative Action (CAPA) items make issue resolution a swift and seamless experience. Send change requests that are reflected throughout your organization for quick results, while detailed effectiveness evaluations indicate where you can optimize your processes.
Book a meeting
Business Process and Regulatory Consulting Services
Tailored specifically to the needs of regulatory and related stakeholders, EXTEDO’s business process and regulatory consulting services are designed to support you through the entire product lifcecycle. Through a series of workshops, our team of experienced consultants will establish your business needs, understand your processes, and help you to define the most appropriate implementation approach.
Education & Training Services
To ensure you get the most out of your purchased solution, we offer detailed training for each product within the EXTEDOpulse solution portfolio. Training sessions are tailored to your individual needs and cover a broad range of technical and regulatory topics. Designed to educate you on how to utilize your EXTEDO solution, our training sessions are conducted either in-house or onsite.
Technical Consulting
Purchasing a new EXTEDO application is the first step to streamlining business and regulatory processes within your organization. However, ensuring correct installation, implementation and integration is a crucial step in the process of deploying your new solution.
Validation Services
Successfully passing an audit from the regulatory agencies requires validated computerized systems. Our team of validation experts has an in-depth knowledge of life sciences business processes, ensuring a tried-and-tested method of system validation. By utilizing the risk-based approach to compliant GxP computerized systems defined within the GAMP 5 standard, we aim to reduce the time, effort, and cost associated with getting your systems up and running.

