
EXTEDOpulse Submission Viewing – Effortlessly view and review eCTD Submissions
An easy-to-use solution for viewing, reviewing and collaborating on submission content.
Save time identifying applications of interest, by easily searching on metadata
Get a centralized overview of regional submissions compiled by local affiliates
Simultaneous collaboration and parallel reviewing during the application compilation process
Are you still using fileshare to review your submission content?
With evolving regulations like eCTD v4, the review process can quickly become an overwhelming challenge without the technical solution.
- I am not able to quickly identify what has changed within the lifecycle of an application
- I struggle meeting the regulator’s procedural timelines during the assessment period
- I can’t easily collaborate and share comments with other stakeholders
- I struggle to have transparency of what our external publishers are working on
- Viewing and reviewing submissions using new or outdated formats is a challenge
- I spend too much time searching for accurate, regulator-approved information
- I cannot use fileshare to review eCTD v4 submissions
- I cannot search my documents based on metadata


Viewing, reviewing and comparing submissions should be effortless.
Application Management
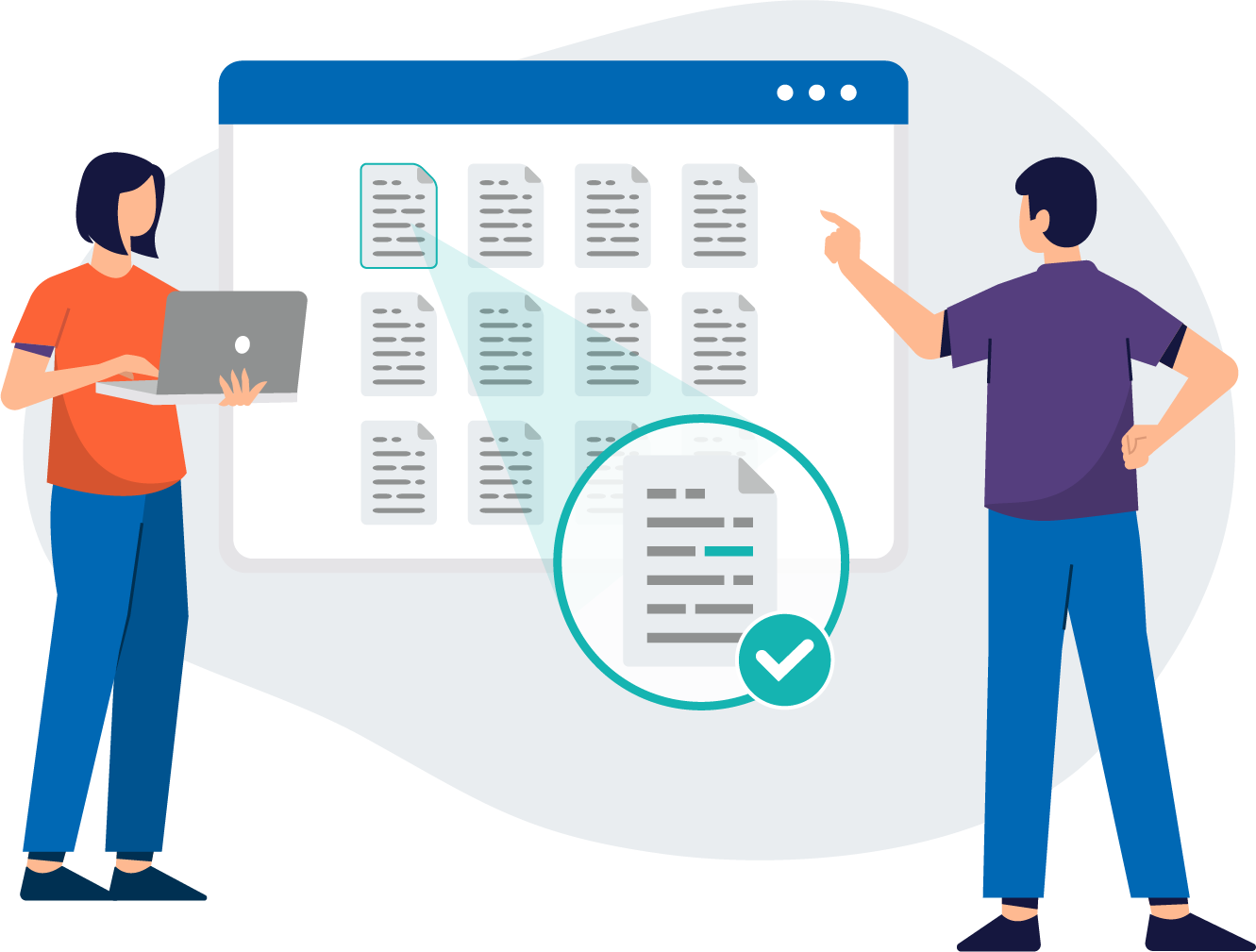
Simply review the entire structure of submissions in a centralized place, using your regular web browser.
Global Application Overview
EXTEDOpulse Submission (Re-)Viewing provides an overview of all folders, documents, and metadata – from all sources and stakeholders, in all formats.
Improved Collaboration
Advanced annotation reviewing helps to collaborate efficiently in real-time.
User experience
Built with simplicity at its heart, EXTEDOpulse reduces complexity and let’s you focus on what’s most important – high-quality applications.



"We were immediately impressed with
EXTEDO’s service and eSUBmanager’s performance. Our team felt that this application was superior to the others we had reviewed."
Your plan to effortless compliance
Schedule a call
Choose a time to meet. We’ll discuss your goals and uncover your challenges with reviewing submissions.
Get a free consultation
Our experienced business and technical team will outline a solution to solve your challenges.
Review submission with ease
With a dedicated reviewing tool you can easily view and comment on all submissions throughout your organization.
Streamline your submission reviews
Stakeholders from various departments within the life sciences industry are often faced with the challenge of reviewing submissions in multiple formats from different sources—without having deep expertise in eCTD. The EXTEDOpulse Submission Management Hub streamlines the viewing, reviewing, and comparison of regulatory submissions, reducing costs by minimizing review time and eliminating friction between teams.


Request your personal EXTEDO Software Demo now!
One consolidated view from multiple sources
The structure of an electronic submission like eCTD v3 and v4 is highly complex and tightly governed. EXTEDOpulse's Submission Management Hub delivers a unified view of all folders, documents, and metadata that form applications and submissions, enabling users to easily visualize their content.

Generative AI Reviewing Assistance
With integrated Generative AI capabilities, EXTEDOpulse’s Submission Management Hub empowers global health authorities and life science users to chat in real time within documents in eCTD format, enabling direct interaction with submission content.
The Generative AI Assistant efficiently retrieves information from a secure, compliant knowledge base. This allows users to save significant hours—what once required searching for specific information, manually locating documents, and reading through them to identify details is now achieved in just seconds.
Each AI-generated response is fully traceable, with clear links to the source document, as well as the related application and sequence number. All data remains securely stored and accessible only for this dedicated use case, ensuring full GDPR compliance and protecting sensitive information.

Comprehensive collaborative reviews
EXTEDOpulse Submission Viewing enables optimized viewing and mark-up without the need to download the entire document. In addition, its collaborative mark-up tools enable suggestions to be made and shared in real-time, without requiring direct edits on the original content.
At the document level, stakeholders can work together simultaneously—commenting, checking documents, and comparing lifecycle versions to accelerate submission reviews across departments and global locations. Performance-tuned rendering ensures even large files load quickly, while visual side-by-side comparison clearly highlights updates, deletions, and additions. Users can annotate a wide range of content formats, including PDFs, Word documents, and videos, ensuring every detail is captured and discussed. The built-in “Where Used” feature instantly reveals document relationships across multiple medicinal product applications, making it easier than ever to track impact and maintain consistency.
Streamlined Master Data Across all Hubs
Using multiple Hubs within EXTEDOpulse, reviewers gain transparent access to far more than just applications—they have a complete overview of regulatory activities, submission content plans, document attributes, and applications. Reusing master data across all Hubs not only improves data quality but also ensures alignment with life sciences organizations’ data governance policies.
Some of our Customers




Contact us
Business Process and Regulatory Consulting Services
Tailored specifically to the needs of regulatory and related stakeholders, EXTEDO’s business process and regulatory consulting services are designed to support you during and after your eCTD submissions. Through a series of workshops, our team of experienced consultants will establish your business needs, understand your processes, and help you to define the most appropriate implementation approach.
Education & Training Services
To ensure you get the most out of your purchased solution, we offer detailed training for each product within the EXTEDOpulse solution portfolio. Training sessions are tailored to your individual needs and cover a broad range of technical and regulatory topics. Designed to educate you on how to utilize your EXTEDO solution, our training sessions are conducted either in-house or onsite.
Technical Consulting
Purchasing a new EXTEDO application is the first step to streamlining business and regulatory processes within your organization. However, ensuring correct installation, implementation and integration is a crucial step in the process of deploying your new solution.

.png)