
White paper:
Simplify your USA FDA eCTD submissions
Navigating USA FDA eCTD requirements: Avoid rejections and ensure compliance. USA FDA submission challenges leave teams feeling...
- Overwhelmed by USA FDA-specific Module 1 requirements and variations
- Confused by the technical details of validation criteria
- Worried about costly submission rejections
- Struggling to integrate compliance into current workflows
We understand these pressures and are here to help you overcome them.
Download the eCTD compliance white paper to avoid rejections and simplify submissions.
Download the white paper
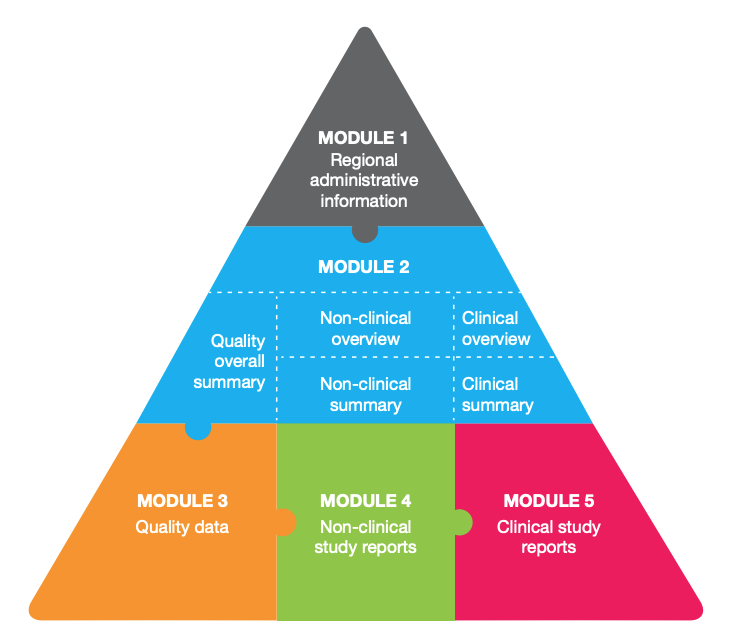
Master USA FDA Module 1 requirements
Our detailed white paper helps you understand the unique role of USA FDA-specific Module 1. This document covers advanced strategies, updates, and ways to optimize your regulatory submission processes.
USA FDA eCTD validation criteria
Learn how to align your submission with USA FDA validation standards to avoid common rejection errors and delays.
Strategic planning for USA FDA eCTD submissions
Discover the essential steps to successfully plan, execute, and validate eCTD projects specifically for USA FDA compliance.
Choosing the right USA FDA eCTD software
Equip yourself with the right questions to evaluate software solutions that streamline USA FDA submissions and ensure compliance with 21 CFR Part 11.
Avoiding USA FDA submission rejections
Gain actionable insights into the most common causes of USA FDA rejections and strategies to prevent them.
Fill in the form to master USA FDA eCTD compliance and stay ahead in the dynamic field of regulatory submissions.
Our Customers





